Understanding the Impact of Generative AI on Drug Discovery: Trends and Insights
Explore specialized Generative AI models in drug discovery, their workings, real-world applications, and emerging trends shaping the industry's future.
7/25/2025
artificial intelligence
9 mins
Generative AI models are introduced with a motivation to make operations smoother, streamlined, and efficient. With all the advancements and innovations coming in, generative AI is now contributing to making drug discovery research and development efficient. Helping to minimize the research and production time from years to a few months.
Traditionally, drug discovery is a resource and effort-intensive task that requires years for research and testing. In today's evolving world, we are witnessing widespread outbreaks of deadly diseases. Evidently, the whole traditional process of making production-ready drugs is inefficient for catering to the demands of such fast-paced, evolving diseases.
Here integration of a generative AI solution can prove to be revolutionary for the drug discovery application. In our blog, we will explore how generative AI in drug discovery can be impactful, which generative AI models can contribute to drug discovery applications, alongside learning about the future of generative AI in drug discovery.
Gen AI in Drug Discovery
Generative AI in drug discovery uses machine learning and AI models to generate novel molecules, drug candidates, or biological data by learning and understanding from the existing dataset. These generative models don't just work to analyze data, but create new, optimized, and useful drug compounds that meet the particular disease need.
How can Gen AI impact drug discovery?
The fast outbreak of COVID-19 in 2020 led to the loss of more than 7 million lives globally. This major outbreak required rapid drug research and development to help reduce the spread and eventually cure the disease to save lives.
For scenarios like this, a generative AI-driven solution could play a critical role in bridging the gap between research and development by learning and analyzing complex data patterns and eventually generating more useful drug compounds. Below, we have explained how generative AI impacts drug discovery by making the process faster, streamlined, and more efficient than ever.
| Drug Discovery Stage | Traditional Time | With Gen AI | Speedup |
|---|---|---|---|
| Hit identification | 6 –12 months | 1 – 7 days | ~10–100x faster |
| Lead optimization | 1-2 years | 2–8 weeks | ~5–20x faster |
| De novo molecule design | 6–24 months | 5–120 minutes | ~1000x faster |
| ADMET prediction | 3–6 months (lab testing) | Seconds – minutes | ~10x faster |
| Target-protein interaction modeling | 2–8 weeks | 2–10 minutes (AlphaFold) | ~100x faster |
| Virtual screening | 3–6 months | 4–12 hours | ~100x faster |
Impact of Gen AI in Drug Discovery Application
So, such an AI tool can perform all the steps for drug discovery more efficiently. This tool autonomously handles all these jobs mentioned below in a more rapid and optimized way, making cure fast and effective, while reducing the spread.
- Hit identification
- Lead optimization
- De novo molecule design (Designing Brand-New Molecules)
- ADMET( Absorption, Distribution, Metabolism, Excretion, Toxicity) prediction
- Target-protein interaction modeling
- Virtual screening
Gen AI Models for Drug Discovery with Application
Generative AI models hold great potential for revolutionizing the drug discovery application. This can handle the whole drug discovery lifecycle more intelligently and efficiently. Below, we have explained how these generative AI models work:
1. VAE
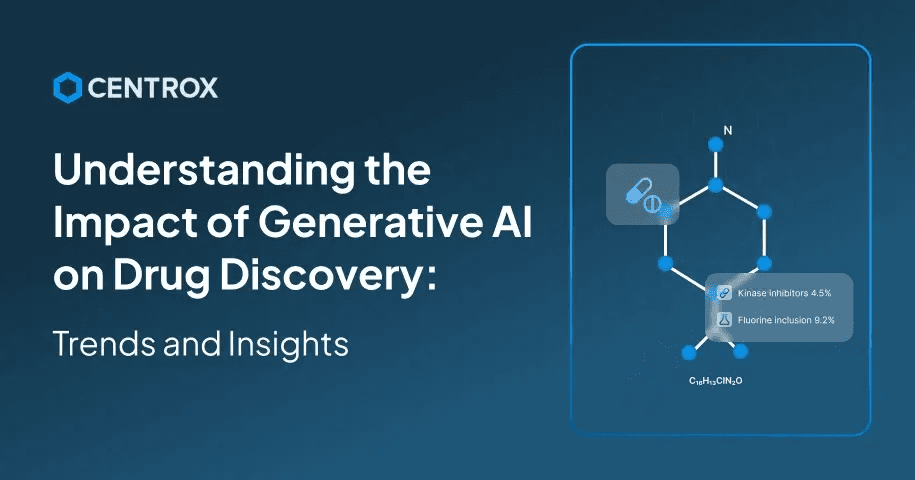
Variational Auto Encoders are a type of generative AI model that can make a significant contribution to drug discovery applications. This model can provide an intelligent approach for handling all the steps involved in drug discovery. For your help, we have explained the step-wise procedure of how VAE works for the dug discovery use case.
- Input Molecules: For input, taking input as SMILES strings or molecular graphs, Molecules from datasets like ChEMBL or ZINC are used.
- Encoder(Compressing Information): Then the encoder works to compress the information by mapping the molecule into a probabilistic latent vector space that represents the chemical feature.
- Latent Space(Chemical Idea Space): The latent space extends a vector space where closely related molecules can cluster together, allowing interpolation and random sampling for new candidates.
- Decoder (Creating New Molecules): Afterwards, the decoder works to reconstruct vectors from the latent space into valid, novel molecular structures.
- Optimization: The latent space can then be further guided to generate molecules with specific properties using reinforcement learning or predictive models.
Industry Application
Insilico Medicine: designs a novel DDR1 kinase inhibitor in under 46 days.
2. GAN’S
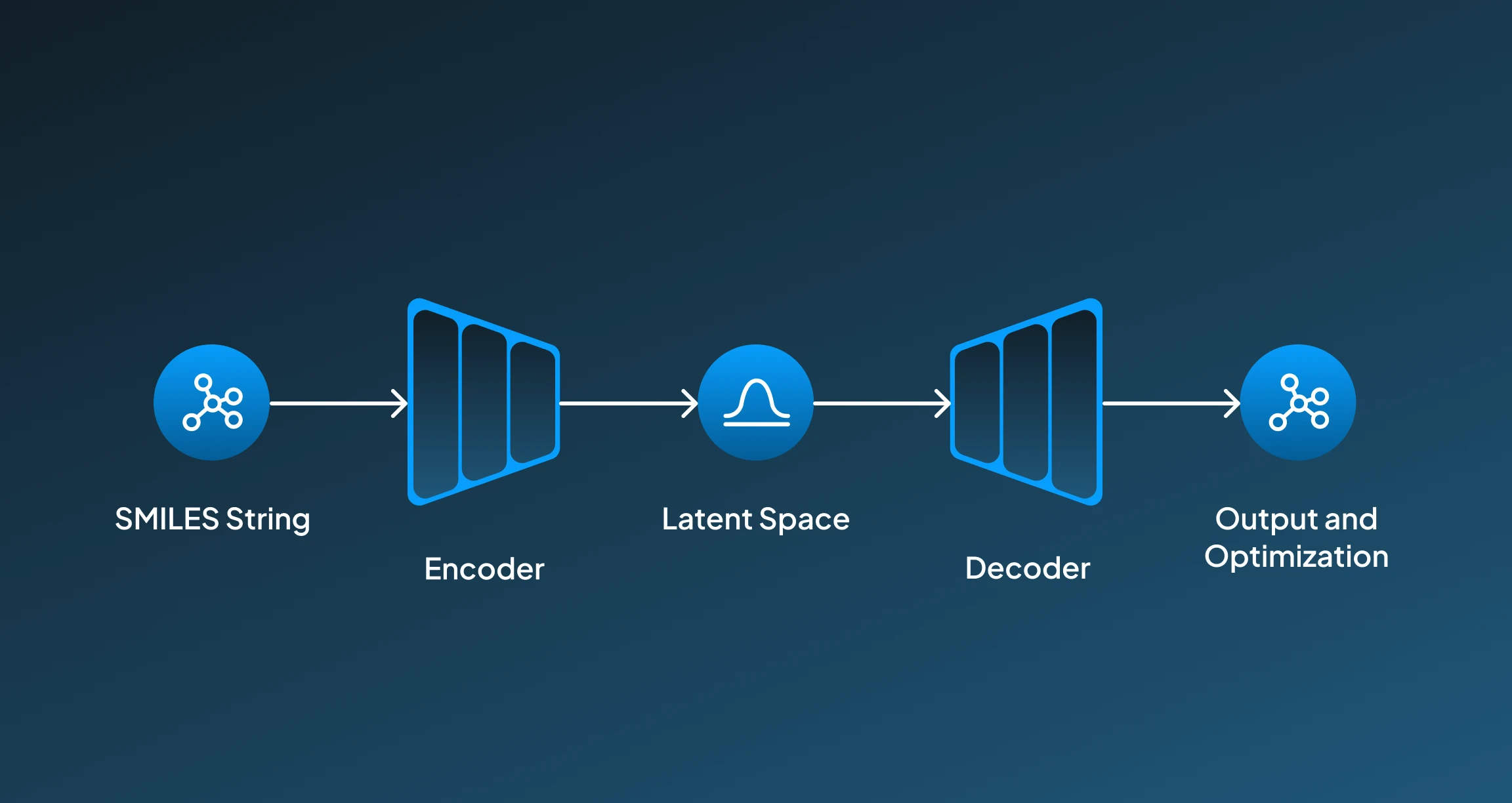
Generative Adversarial Network is one class of generative AI models that has two competing networks working against each other. For a high-impact application like drug discovery, GAN enables an optimized approach to generating molecules that resemble known bioactive compounds. Here, a step-wise breakdown of how GANs work for drug discovery:
- Input Molecules: GANs are trained on particular molecular representations like SMILES strings or molecular graphs, which are often sourced from datasets like ZINC or PubChem.
- Generator(Creates Fake Molecules): Here, the generator network functions for creating new molecular structures that resemble existing ones from the training dataset.
- Discriminator( Validating Molecules): The discriminator then works for evaluating whether the generated molecule by the generator is real (from the dataset) or fake (generated), helping the generator to improve.
- Latent Space Exploration: Then the training progresses, encouraging the generator learns to explore the latent molecular space for producing highly realistic, impactful, and diverse drug-like molecules.
- Optimization: We can also train the molecules to adjust the final compound according to particular properties like low toxicity, high solubility, or binding affinity.
Industry Application
MoIGAN: generates small molecular graphs with property optimization, widely referenced in drug design research.
3. Diffusion Model
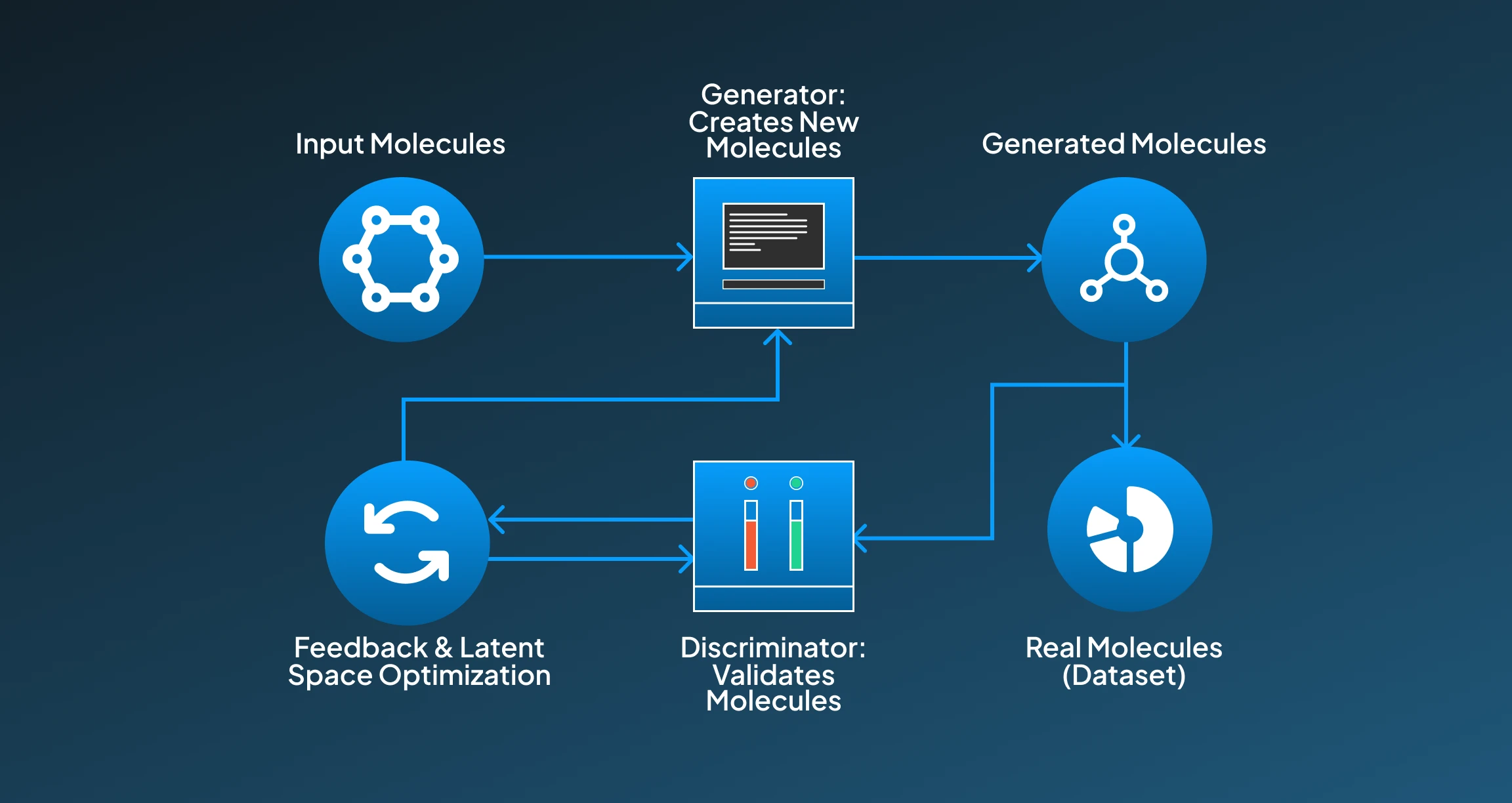
Diffusion models are a new class of generative AI models that work by transforming random noise into structured output. For drug discovery applications, these diffusion models possess great ability to generate high-fidelity and diverse chemical structures. Below, we have provided a procedural breakdown for how the diffusion model functions for drug discovery applications:
- Input Molecules: For the training process, known molecules (e.g., from ChEMBL or ZINC) are utilized and often represented as 3D graphs or coordinate-based structures.
- Forward Diffusion Process; Then, for the forward diffusion process, molecules are slowly changed into noise through a defined process, enabling the model to learn how molecules degrade.
- Reverse Diffusion Process: The model then initiates learning to reverse this noise into reconstructed valid molecules through a denoising process.
- Latent Space and Noise Sampling: Furthermore, the molecule generation then starts from pure noise, the it works by sampling points in the latent space and incrementally refining them into realistic drug candidates.
- Optimization: Additionally, these diffusion models can be directed by property prediction networks to bias generation toward molecules with desirable therapeutic contributions.
Industry Application
GeoDiff: generates 3D molecular conformations and ligand-protein binding poses, offering precise structure-based drug discovery tools.
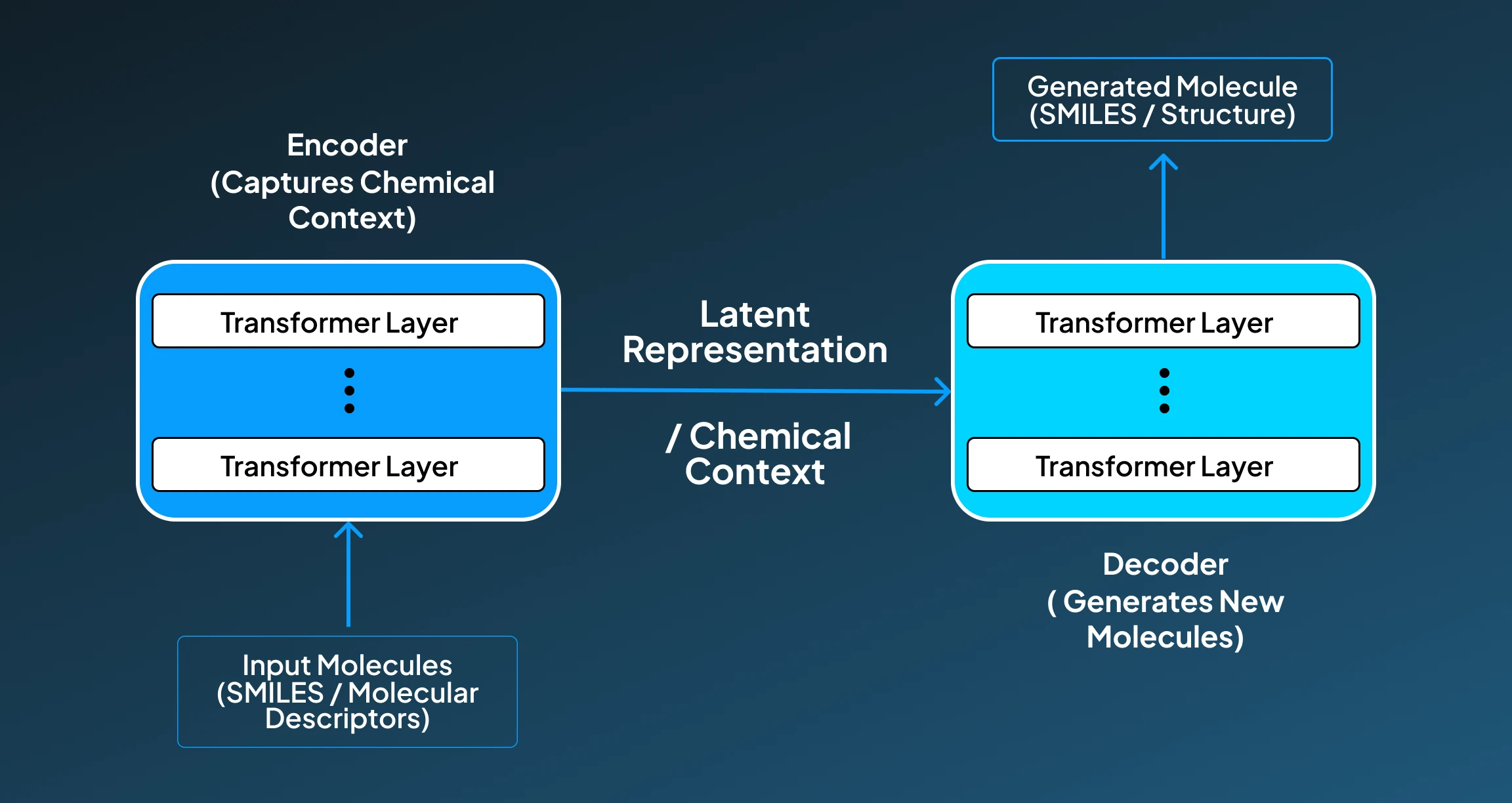
4. Transformer-Based Architecture
The Transformers sequence-based generative AI model shows great ability to capture long-range dependencies. For applications like drug discovery, transformer models can contribute to tasks like molecule generation, retrosynthesis, and property prediction. To help you get a better overview, we have provided a step-by-step breakdown of how the transformer model functions for a drug discovery application:
- Input Molecules: The Transformers model starts its work by taking input as SMILES strings or tokenized molecular descriptors from datasets like ZINC, ChEMBL, or BindingDB.
- Encoding Chemical Context: Then the encoder processes the entire input molecule as a sequence and begins to capture contextual information across all atoms and bonds using attention mechanisms.
- Latent Representation: Followed by this, the model starts building a rich internal representation of the molecule, enabling tasks like generation, translation (e.g., retrosynthesis), or property prediction.
- Decoding / Generation: Finally, the decoder or auto-regressive head generates new SMILES strings or sequences representing novel molecules, atom by atom or token by token.
- Optimization: For improving the performance, we can fine-tune or condition to generate molecules with specific characteristics (e.g., binding to a target protein, non-toxic, BBB permeable).
Industry Application
ChemBERT: Is an industry-available application for property prediction and molecule generation.
Future of Gen AI in Drug Discovery
As we move ahead, we can predict rising expectations of users from generative AI, especially for drug discovery applications. With the current and ongoing efforts, we can safely say that generative AI definitely has the potential to offer more for drug discovery and production. Below, we have highlighted some futuristic innovations that Gen AI can offer for Drug discovery:
1. End-to-End AI-Driven Drug Design
- What’s Coming: In the future, we can anticipate that generative AI will be able to entire drug discovery life cycle process. It will be able to oversee target identification and molecular generation, optimization, with minimal human intervention.
- Challenge: Ensuring regulatory compliance and scientific validation of AI-generated drug candidates remains a critical hurdle before full-scale adoption.
- Impact: With this advancement, it can significantly minimize the discovery time and cost, enabling faster go-to-market drugs for new therapies.
2. Personalized Drug Generation
- What’s Coming: A generative AI-based solution can create customized drugs based on individual genetic, proteomic, or disease-specific data. It can design a compound drug that has shown evidence-based good results
- Challenge: Accessing and securely managing high-quality patient data while maintaining privacy is a major challenge in personalized drug development.
- Impact: Leads to highly targeted treatments, improved efficacy, and fewer side effects for patients.
3. Integration with Lab Automation
- What’s Coming: A generative AI-driven solution can be used for designing molecules, and accordingly synthesizing and testing the prepared drug with the help of automated tools integrated in the drug lab.
- Challenge: High setup costs and the complexity of integrating AI models with existing lab automation infrastructure can slow down adoption.
- Impact: This application aids in creating a rapid, closed-loop system from molecule design to real-world validation, speeding up the preclinical research.
Thoughts to Remember
Generative AI is redefining the scope of drug discovery by introducing an alternative for trial-based processes with intelligent, performance-driven innovation. Whether through VAEs compressing molecular structures into optimized candidates or GANs generating realistic compounds, or diffusion models crafting 3D drug conformations, it is enabling measurable speed, efficiency, and precision.
With its ability to design novel, safe, and effective drugs in weeks instead of years it reflects a transformative leap in pharmaceutical R&D. As these models integrate with lab automation and personalize medicine at scale, the future of drug development is not just faster, it’s smarter, targeted, and significantly more cost-effective than ever before.
Do you want to see how generative AI can accelerate your drug discovery process and deliver smarter, faster results? Book your session today with our experts at Centrox AI, and find out how you can redefine the future with generative AI.

Muhammad Harris
Muhammad Harris, CTO of Centrox AI, is a visionary leader in AI and ML with 25+ impactful solutions across health, finance, computer vision, and more. Committed to ethical and safe AI, he drives innovation by optimizing technologies for quality.
Do you have an AI idea? Let's Discover the Possibilities Together. From Idea to Innovation; Bring Your AI solution to Life with Us!
Your AI Dream, Our Mission
Partner with Us to Bridge the Gap Between Innovation and Reality.
